Ocean CO2 collector could fight global warming and ocean acification
Ocean CO2 collector could fight global warming and ocean acification
mongabay.com
November 20, 2007
Researchers have proposed a geoengineering solution to global warming that involves building a series of water treatment plants that enhance the ability of the ocean to absorb carbon dioxide from the atmosphere by removing hydrochloric acid from seawater by electrolysis. The study is published in the American Chemical Society’s Environmental Science & Technology.
Kurt Zenz House of Harvard University and colleagues say the construction of 100 such water treatment facilities worldwide could serve as a “giant carbon dioxide collector” and reduce 15 percent of global CO2 emissions. 700 plants would offset all CO2 emissions.
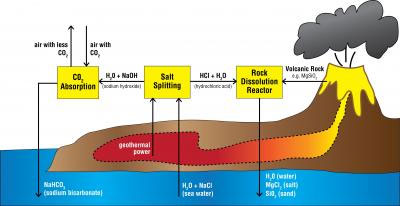
Carbon Dioxide Collector. Illustration depicts how the oceans could be used as a giant carbon dioxide collector to fight global warming. Credit: Courtesy of Kurt House, Harvard University |
The water treatment facilities worldwide “would remove hydrochloric acid from the ocean by electrolysis and neutralize the acid through reactions with silicate minerals or rocks,” according to a statement from the American Chemical Society. “The reaction increases the alkalinity of the ocean and its ability to absorb carbon dioxide from the atmosphere. The process is similar to the natural weathering reactions that occur among silicate rocks but works at a much faster rate.”
The treatment plants could also help stave off damage to marine ecosystems that would otherwise result from increasingly acidic oceans. When carbon dioxide dissolves in water it makes the water more acidic by stripping out carbonate ions, which are essential for marine organisms to build calcium carbonate shells and exoskeletons. Scientists estimate that should CO2 emissions continue to rise at the present rate, by 2100 the amount of carbonate available for marine organisms could drop by 60%. In surface ocean waters, where acidification starts before spreading to the deep sea, there may be too little carbonate for organisms to form shells as soon as 2050. The loss of these small organisms would have a disastrous impact on predators — including salmon, mackerel, herring, cod — that rely on them as a food source and could spell trouble for other species.
While the proposed treatment plants could help protect marine biodiversity and stabilize climate, they would come at a high cost, according to analysis by Biopact, an energy website.
“A range of efficiency scenarios indicates that the process should require 100—400 kJ of work per mol of CO2 captured and stored for relevant timescales. This means the process is energy intensive. The researchers suggest to utilize power from ‘stranded energy sources’ too remote to be useful for the direct needs of population centers,” states Biopact. “But herein lies a problem. If these ‘stranded energy sources’ are fossil fuels, the energy required to desalinate the water may contribute to the release of more carbon dioxide than the method sequesters. The work input required for the overall process is expected to be between 1.5 and 3.5 times higher per unit of CO2 than the work required for postcombustion capture and geologic storage (CCS) of CO2 from a modern coal-fired power plant.”
“The new geoengineering technique will not be able to compete with either [coal + carbon capture] or [biomass and carbon capture and geologic storage]”
CITATION: Kurt Zenz House, Christopher H. House, Daniel P. Schrag, and Michael J. Aziz (2007). Electrochemical Acceleration of Chemical Weathering as an Energetically Feasible Approach to Mitigating Anthropogenic Climate Change. Environmental Science & Technology. Dec. 15














