Carbon dioxide levels threaten oceans regardless of global warming
Carbon dioxide levels threaten oceans regardless of global warming
March 8, 2007
Rising levels of carbon dioxide will have wide-ranging impacts on the world’s oceans regardless of climate change, reports a study published in the March 9, 2007, issue of the journal Geophysical Research Letters
The study, authored by Ken Caldeira from the Department of Global Ecology at the Carnegie Institution at Stanford University and Long Cao and Atul Jain of the University of Illinois, shows the increasing absorption of carbon dioxide is acidifying global oceans, putting sea life at risk.
“Whether you believe in global warming or not, CO2 is going to run havoc in the oceans if unabated, ” warned coauthor Dr. Caldeira. “Temperature increases from climate change affect salinity, circulation, and marine biology. When carbon dioxide dissolves in the ocean, some of it becomes carbonic acid—a corrosive agent, which can eat away shells of important species in the global food chain.”
Oceans worldwide absorbed approximately 118 billion metric tons of carbon between 1800 and 1994 according to a report published last year by scientists at the National Center for Atmospheric Research and NOAA, resulting in increased ocean acidity, which reduces the availability of carbonate ions needed for the production of calcium carbonate structures. In the past, changes in ocean acidity have triggered mass extinction events. According to a study published in the September issue of Geology, dramatically warmer and more acidic oceans may have contributed to the worst mass extinction on record, the Permian extinction. During the extinction event, which occurred some 250 million years ago, about 95% of ocean’s life forms became extinct. The same fate could befall modern day marine life.
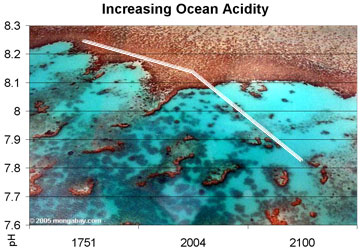
Observed and projected decline in global ocean pH, 1750-2100. Photo by Rhett A. Butler |
Late last year a team of scientists writing in Nature warned that by 2100, the amount of carbonate available for marine organisms could drop by 60%. In surface ocean waters, where acidification starts before spreading to the deep sea, there may be too little carbonate for organisms to form shells as soon as 2050. The loss of these small organisms would have a disastrous impact on predators — including salmon, mackerel, herring, cod — that rely on them as a food source and could spell trouble for other species.
Carbon dioxide is a byproduct of fossil fuels combustion. Scientists estimate that the oceans have soaked up about half of all carbon dioxide produced from fossil fuel emissions over the past 200 years. Had oceans not absorbed this carbon, current atmospheric carbon dioxide would be much higher than the current 381 parts-per-million (ppm)–probably closer to 500-600 ppm say climatologists.
This absorption has made the world’s oceans significantly more acidic since the beginning of the industrial revolution. Research published last year by Mark Jacobson, an assistant professor of civil and environmental engineering at Stanford University, indicated that between 1751 and 2004 surface ocean pH dropped from approximately 8.25 to 8.14. James Orr of the Climate and Environmental Sciences Laboratory further estimated that ocean pH levels could fall another 0.3 – 0.4 units by 2100.
The new Geophysical Research Letters paper confirms Orr’s forecasts, projecting a 0.31 drop in pH units by the end of this century “if CO2 emissions continue on their current trajectory to stabilize at atmospheric CO2 concentrations at 1000 parts per million.” Caldeira says their new model shows that overall temperature change won’t have much effect on ocean acidity.
“Since surface temperature increases affect how carbon is broken down in seawater, we wanted to quantify how the acidity of the water would be affected by temperature increases from CO2 emissions,” he explained in a statement. “We found that the pH, or acidity, of the water wasn’t significantly affected regardless of how much warming occurs over the next decades and centuries.”
Their model further showed that a doubling of carbon dioxide levels would produce a pH decline of 0.48-0.51 units by the year 2500.
“Ocean acidification threatens all marine organisms that use calcium carbonate to make their shells,” Caldeira added. “However even as the planet warms, our study shows that we can help the ecological balance in the oceans by curbing CO2 emissions now by using wind, solar, nuclear power, and other alternative energy sources.”
The findings suggest strategies that focus solely on moderating global temperatures, while neglecting carbon dioxide emissions (i.e. injecting sulfates into the upper atmosphere to reflect sunlight) will not counter the negative impact of ocean acidity.
CITATION: L. Cao, Caldeira, K., and Jain, A.K., “Effects of carbon dioxide and climate change on ocean acidification and carbonate mineral saturation,” Geophysical Research Letters (GRL) paper 10.1029/2006GL028605, 2007.
This article uses quotes from a Carnegie Institution news release along with excerpts from previous mongabay.com articles written by Rhett.














