Aquarium Fish May be the Key to New Therapies for Birth Defects
Aquarium Fish May be the Key to New Therapies for Birth Defects
Vanderbilt University
August 9, 2006
A humble aquarium fish may be the key to finding therapies capable of preventing the structural birth defects that account for one out of three infant deaths in the United States today
That is one of the implications of a new study published online August 8 in the journal Cell Metabolism. The paper describes a number of striking parallels between a rare but fatal human birth defect called Menkes disease and a lethal mutation in a small tropical fish called the zebrafish that has become an important animal model for studying early development.
Zebrafish are easy and inexpensive to raise and lay eggs that are transparent and develop outside the body. Much of the zebrafish genome has been sequenced, allowing researchers to identify human versions of zebrafish genes and vice versa. These qualities make the zebrafish exceptionally handy in studying the complex relationship between genes and nutrition during development, a puzzle that has stood in the way of developing effective treatments for birth defects.
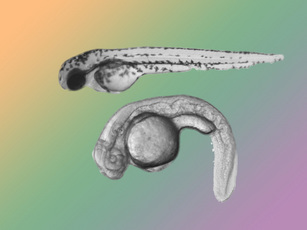
A normal, wildtype zebrafish embryo is shown above an embryo with the calamity mutation. The calamity mutant impairs so many aspects of normal development that it falls apart in about two days. Image courtesy of the Solnica-Krezel Laboratory. |
“This is a proof of concept that we can use the zebrafish to finally understand the role that maternal nutrition plays in causing structural birth defects and develop new treatments that can prevent them,” said co-author Jonathan Gitlin, the Helene B. Robertson Professor of Pediatrics at Washington University in Saint Louis. Collaborating in the study were Professor of Biological Sciences Lilianna Solnica-Krezel, postdoctoral fellow Thomas P. Wilm and graduate student Chunyue Yin from Vanderbilt University along with Associate Professor of Genetics Stephen L. Johnson and graduate student Bryce A. Mendelsohn from Washington University in St. Louis.
For many years, scientists have relied on the mouse and the frog Xenopus laevis as research models for vertebrates (animals with backbones), but zebrafish have an important advantage for studying early development: It is possible for researchers to watch the changes that take place in their embryos from the moment of fertilization. The small fish have another advantage for studying the role of nutrition: Scientists can precisely regulate the nutrients that the embryo receives largely free from maternal influence.
In the paper, the researchers describe the discovery of a mutation in the zebrafish that disrupts the distribution of the critical nutrient copper within the fish cells and causes defects that are remarkably similar to those observed in children suffering from Menkes kinky hair disease, which, in its most severe form, causes degeneration and death within two to three years after birth.
“We found this mutation about two and a half years ago,” said Solnica-Krezel. “Because it impairs so many aspects of normal development and causes the embryo to fall apart in two days, we named it Calamity.'” Six months later, she heard a talk that Gitlin gave at a scientific meeting about the results of exposing zebrafish embryos to a chemical agent that disrupts copper metabolism. She was struck by the similarity between his results and those produced by Calamity, so she approached him and they decided to collaborate.
“Lila and the other zebrafish geneticists have created all these wonderful tools,” said Gitlin. “I feel like a cook that walks into this wonderfully equipped kitchen and realizes there all these wonderful recipes that he can prepare!”
The researchers explored the effect on embryo development of varying the amount of copper available and disabling different pieces of the molecular mechanisms that cells use to handle this potentially toxic material. The consequences were quite dramatic because copper-containing enzymes play a number of critical roles in the life of the cell. They are involved in the process by which a cell burns sugars and disposes of the carbon dioxide and water byproducts. They are required to produce pigmentation and develop connective tissue. These “cuproenzymes” are also necessary for creating the neurotransmitters essential for brain function.
The researchers discovered that the Calamity mutation occurs in a gene that codes for a key copper distribution enzyme, labeled Apt7a. This is a very large and complicated protein that sits in a cell structure called the Golgi complex, which manufactures a large variety of enzymes that function both inside and outside of the cell. Apt7a also extends outside of the cell and its primary function is to bring copper atoms into the cell and transport them to the Golgi for incorporation into various enzymes. They have determined that the Calamity mutation occurs in a location that totally inactivates the enzyme.
To determine whether the disruption in copper distribution was confined to individual cells, the researchers inserted normal cells into calamity embryos. The fact that the “wild-type” cells functioned normally helped to explain why attempts to treat Menkes with copper supplements has only proven beneficial in the milder versions of the disease. It showed that the problem was not lack of copper per se, but an inability of cells to import the copper that they need to produce key enzymes.
Next, the scientists established that the zebrafish gene is a close cousin of the human Menkes gene. They injected the human Menkes enzyme into Calamity embryos and found that it works almost as well as the fish enzyme and rescues embryos from a number of the mutant’s defects.
That demonstrated just how similar the molecular mechanisms for handling copper are in the zebrafish and in humans. This didn’t come as a big surprise because the researchers know that evolution tends to be highly conservative. That is, when nature finds an effective way to accomplish a life-critical operation, it tends to keep using it. Handling metal atoms like copper is one such operation. These atoms are essential but can be very destructive if they get loose within the cell.
“The fact that injecting the human gene can rescue the zebrafish embryo is also an example of gene therapy that works really well, at least temporarily in the fish,” adds Solnica-Krezel.
The zebrafish also makes it possible to pursue another, even more promising approach for developing a treatment for Menkes: Testing hundreds or thousands of compounds to see if any can restore proper copper-handling in Calamity mutants. If such a drug can be found, it would be a strong candidate for treating the disorder in human embryos.
“The zebrafish is the first animal model that allows us to watch the process of early vertebrate development and manipulate it. There is no reason why the same approach that we have used with copper cannot work for other nutrients as well,” said Gitlin.
In fact, applying this approach to other nutrients and other types of birth defects is the goal of the Children’s Discovery Institute, a major new initiative that Gitlin is heading as a joint venture between the St. Louis Children’s Hospital and Washington University.
This research was funded by grants from the National Institutes of Health.
This is a modified news release from Vanderbilt University.













