Scientists develop cheap catalyst for hydrogen production from biofuels
Scientists from Ohio State University have developed a very cheap non-precious metal catalyst that converts biofuels like ethanol into hydrogen with an efficiency of up to 90%. This development opens up a future of decentralised, on-the-spot hydrogen production for use in fuel cell cars. What is more, it makes the prospect of a carbon-negative transportation fuel more realistic.
The rationale behind converting biofuels to hydrogen is simple: you no longer need an expensive hydrogen transportation infrastructure, because you can transport the fuel safely in the form of the biofuel and turn it into hydrogen wherever you want; using hydrogen in fuel cells is also far more efficient than using biofuels in internal combustion engines.
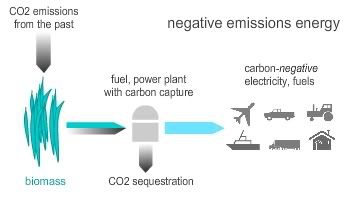
Best of all, when the carbon dioxide that is released during the conversion process is captured and sequestered, a truly carbon-negative fuel is obtained. The more you were to use of this fuel, the more you were to combat climate change, because you would be actively removing CO2 from the atmosphere (earlier post, and see schematic).
Umit Ozkan, professor of chemical and biomolecular engineering at Ohio State University, says that the new catalyst is much less expensive than others being developed around the world, because it does not contain precious metals, such as platinum or rhodium. Rhodium is used most often for this kind of catalyst, and it costs around $9,000 an ounce. The new catalyst costs around $9 a kilogram - that's about 35,000 times less.
The new catalyst allows us to over come the many practical issues that need to be resolved before we can use hydrogen as fuel - how to make it, how to transport it, how to create the infrastructure for people to fill their cars with it.
The new dark gray powder is made from tiny granules of cerium oxide - a common ingredient in ceramics - and calcium, covered with even smaller particles of cobalt. It produces hydrogen with 90 percent efficiency at 660 degrees Fahrenheit (around 350 degrees Celsius) - a low temperature by industrial standards.
Whenever a process works at a lower temperature, that brings energy savings and cost savings. Also, if the catalyst is highly active and can achieve high hydrogen yields, one doesn’t need as much of it. That will bring down the size of the reactor, and its cost:
 energy :: sustainability :: ethanol :: biodiesel :: biomass :: bioenergy :: biofuels :: biohydrogen :: hydrogen :: efficiency :: catalyst :: carbon-negative ::
energy :: sustainability :: ethanol :: biodiesel :: biomass :: bioenergy :: biofuels :: biohydrogen :: hydrogen :: efficiency :: catalyst :: carbon-negative ::
The process starts with a liquid biofuel such as ethanol, which is heated and pumped into a reactor, where the catalyst spurs a series of chemical reactions that ultimately convert the liquid to a hydrogen-rich gas.
One of the biggest challenges the researchers faced was how to prevent "coking" -- the formation of carbon fragments on the surface of the catalyst. The combination of metals - cerium oxide and calcium - solved that problem, because it promoted the movement of oxygen ions inside the catalyst. When exposed to enough oxygen, the carbon, like the biofuel, is converted into a gas and gets oxidized; it becomes carbon dioxide.
At the end of the process, waste gases such as carbon monoxide, carbon dioxide and methane are removed, and the hydrogen is purified. To make the process more energy-efficient, heat exchangers capture waste heat and put that energy back into the reactor. Methane recovered in the process can be used to supply part of the energy.
Though this work was based on converting ethanol, Ozkan's team is now studying how to use the same catalyst with other liquid biofuels. Her coauthors on this presentation included Ohio State doctoral students Hua Song and Lingzhi Zhang.
The research was funded by the U.S. Department of Energy.
References:
Ohio State University: A Better Way to Make Hydrogen from Biofuels - August 20, 2008.
Biopact: The strange world of carbon-negative bioenergy: the more you drive your car, the more you tackle climate change - October 29, 2007
The rationale behind converting biofuels to hydrogen is simple: you no longer need an expensive hydrogen transportation infrastructure, because you can transport the fuel safely in the form of the biofuel and turn it into hydrogen wherever you want; using hydrogen in fuel cells is also far more efficient than using biofuels in internal combustion engines.

Umit Ozkan, professor of chemical and biomolecular engineering at Ohio State University, says that the new catalyst is much less expensive than others being developed around the world, because it does not contain precious metals, such as platinum or rhodium. Rhodium is used most often for this kind of catalyst, and it costs around $9,000 an ounce. The new catalyst costs around $9 a kilogram - that's about 35,000 times less.
The new catalyst allows us to over come the many practical issues that need to be resolved before we can use hydrogen as fuel - how to make it, how to transport it, how to create the infrastructure for people to fill their cars with it.
Our research lends itself to what's called a 'distributed production' strategy. Instead of making hydrogen from biofuel at a centralized facility and transporting it to gas stations, we could use our catalyst inside reactors that are actually located at the gas stations. So we wouldn't have to transport or store the hydrogen - we could store the biofuel, and make hydrogen on the spot. - Professor Umit OzkanThe catalyst is inexpensive to make and to use compared to others under investigation worldwide. Those others are often made from precious metals, or only work at very high temperatures. Precious metals have high catalytic activity and - in most cases - high stability, but they're also very expensive. The scientists' goal from the outset was to come up with a precious-metal-free catalyst, one that was based on metals that are readily available and inexpensive, but still highly active and stable. This sets Ozkan's team apart from most of the other groups in the world.
The new dark gray powder is made from tiny granules of cerium oxide - a common ingredient in ceramics - and calcium, covered with even smaller particles of cobalt. It produces hydrogen with 90 percent efficiency at 660 degrees Fahrenheit (around 350 degrees Celsius) - a low temperature by industrial standards.
Whenever a process works at a lower temperature, that brings energy savings and cost savings. Also, if the catalyst is highly active and can achieve high hydrogen yields, one doesn’t need as much of it. That will bring down the size of the reactor, and its cost:
 energy :: sustainability :: ethanol :: biodiesel :: biomass :: bioenergy :: biofuels :: biohydrogen :: hydrogen :: efficiency :: catalyst :: carbon-negative ::
energy :: sustainability :: ethanol :: biodiesel :: biomass :: bioenergy :: biofuels :: biohydrogen :: hydrogen :: efficiency :: catalyst :: carbon-negative :: The process starts with a liquid biofuel such as ethanol, which is heated and pumped into a reactor, where the catalyst spurs a series of chemical reactions that ultimately convert the liquid to a hydrogen-rich gas.
One of the biggest challenges the researchers faced was how to prevent "coking" -- the formation of carbon fragments on the surface of the catalyst. The combination of metals - cerium oxide and calcium - solved that problem, because it promoted the movement of oxygen ions inside the catalyst. When exposed to enough oxygen, the carbon, like the biofuel, is converted into a gas and gets oxidized; it becomes carbon dioxide.
At the end of the process, waste gases such as carbon monoxide, carbon dioxide and methane are removed, and the hydrogen is purified. To make the process more energy-efficient, heat exchangers capture waste heat and put that energy back into the reactor. Methane recovered in the process can be used to supply part of the energy.
Though this work was based on converting ethanol, Ozkan's team is now studying how to use the same catalyst with other liquid biofuels. Her coauthors on this presentation included Ohio State doctoral students Hua Song and Lingzhi Zhang.
The research was funded by the U.S. Department of Energy.
References:
Ohio State University: A Better Way to Make Hydrogen from Biofuels - August 20, 2008.
Biopact: The strange world of carbon-negative bioenergy: the more you drive your car, the more you tackle climate change - October 29, 2007
 --------------
--------------
 Mongabay, a leading resource for news and perspectives on environmental and conservation issues related to the tropics, has launched Tropical Conservation Science - a new, open access academic e-journal. It will cover a wide variety of scientific and social studies on tropical ecosystems, their biodiversity and the threats posed to them.
Mongabay, a leading resource for news and perspectives on environmental and conservation issues related to the tropics, has launched Tropical Conservation Science - a new, open access academic e-journal. It will cover a wide variety of scientific and social studies on tropical ecosystems, their biodiversity and the threats posed to them.









2 Comments:
I would take this one step further: Miniaturize the processor and put it onboard the moving vehicle. That way you would carry the liquid biofuel such as pure ethanol. This would eliminate the need to carry special tanks filled with ultra high pressure compressed hydrogen. Hydrogen may be here now.
Our company, Limnia, Inc. (http://www.limnia.com) has resolved almost all of the issues associated with hydrogen infrastructure. We store hydrogen in patented, solid-state, non-pressurized canisters that are safe, efficient, better than many battery solutions, use common carriers already built out globally and can recharge a car in seconds via hot-swap. Our many hydrogen generation partners have shown methods, this year, to make hydrogen from water or organic waste for highly efficiency energy ratios.
Cindy Lewis
Post a Comment
Links to this post:
Create a Link
<< Home