Researchers visualize complex pigment mixtures in living cells: technique offers new insights into photosynthesis at molecular level
 In a technical advance that could allow researchers to watch cells as they act during the process of photosynthesis, scientists funded by the U.S. Department of Energy have developed a method that extends the power of fluorescence-mediated bio-imaging to see discrete pigments inside live cells of bacteria. The method is providing fresh insights into what happens on a molecular level during photosynthesis. It also promises to provide important information about the inner workings of cells as they engage in the photosynthesis process of collecting sunlight and turning it into chemical energy. The new tool is set to become an integral part of "systems biology".
In a technical advance that could allow researchers to watch cells as they act during the process of photosynthesis, scientists funded by the U.S. Department of Energy have developed a method that extends the power of fluorescence-mediated bio-imaging to see discrete pigments inside live cells of bacteria. The method is providing fresh insights into what happens on a molecular level during photosynthesis. It also promises to provide important information about the inner workings of cells as they engage in the photosynthesis process of collecting sunlight and turning it into chemical energy. The new tool is set to become an integral part of "systems biology".The new capacity to gather information about photosynthesis this way could be valuable in helping researchers fine tune the bacteria for specific purposes, said Wim Vermaas, a professor in Arizona State University's (ASU) School of Life Sciences, member of the university's Center for Bioenergy and Photosynthesis and lead author.
The article titled "In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells" was published in this week’s online Early Edition of the Proceedings of the National Academy of Sciences. The ASU researchers worked with scientists from Sandia National Laboratories, Albuquerque, on the new method.
The method is based on fluorescence imaging to discern the different pigments of bacteria that are engaged in photosynthesis. Fluorescence is a property where some compounds emit a characteristic glow when excited by specific wavelengths of light. Up until now, current fluorescent methods have had a hard time discerning compounds with similar pigments and fluorescence characteristics, hampering the ability of researchers to know exactly what is going on inside a cell.
Confocal fluorescence microscopy has proven to be an excellent method to localize pigments in cells as long as there is little spectral overlap between different fluorescing pigments. The new method, "hyperspectral fluorescence imaging", greatly pushes the boundaries of this technique, and can separately localize pigments with similar fluorescence spectra. The researchers employed an advanced image analysis method that was developed at Sandia Labs.
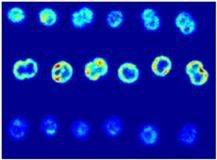
This is a new tool in our tool box, and a very good one at that. [...] This is an analysis method that is superior to what commercial analysis systems can do. It tells you where the fluorescent materials are in the cell, what the fluorescence of each material looks like and how much is present even if the fluorescence properties look like each other. - Professor Wim Vermaas, lead authorThe initial study focused on localization of pigments in a cyanobacterium, a specific type of bacteria of interest to the team. With the method, they showed that photosynthesis-related pigments (chlorophylls, phycobilins and carotenoids) can be localized in vivo in cells of the cyanobacterium Synechocystis sp. PCC 6803 through deconvolution of individual fluorescence emission spectra in small (0.03 cubic micrometer) volumes by means of hyperspectral confocal fluorescence imaging:
 energy :: sustainability :: biomass :: bioenergy :: biofuels :: plant cell :: metabolism :: photosynthesis :: cyanobacteria :: bio-imaging ::
energy :: sustainability :: biomass :: bioenergy :: biofuels :: plant cell :: metabolism :: photosynthesis :: cyanobacteria :: bio-imaging :: The method allows us to push the resolution limits of confocal fluorescence microscopy, particularly when there are mixtures of different fluorescent compounds with relatively similar spectra. In the specific case of cyanobacteria, it enables the detection of different pigments relative to each other in the cell, and we were able to localize the two different photosystems in the cell relative to each other, along with other pigments. - Professor VermaasUsing the technique, the researchers report that results obtained indicate a heterogeneous composition of thylakoid membranes in cyanobacteria: Phycobilin emission was most intense along the periphery of the cell whereas chlorophyll fluorescence was distributed more evenly throughout the cell, suggesting that fluorescing phycobilisomes are more prevalent along the outer thylakoids. Carotenoids were prevalent in the cell wall and also were present in thylakoids.
Two chlorophyll fluorescence components also were resolved: the short-wavelength component originated primarily from photosystem II and was most intense near the periphery of the cell; and the long-wavelength component that is attributed to photosystem I, because it disappears in mutants lacking this photosystem, was of higher relative intensity toward the inner rings of the thylakoids. Together, the results suggest compositional heterogeneity between thylakoid rings, with the inner thylakoids enriched in photosystem I.
Vermaas said this means that even in a simple and small cyanobacterial cell (about a hundred fold smaller than can be seen by the human eye) there is an exquisite functional division of labor between membranes inside the cell, with different processes in photosynthesis in different areas of the membranes.
We found that the two photosystems are not fully co-localized in thylakoids in the cell, even though thylakoids ‘look all the same’ in electron micrographs. Based on this, the way the cells probably work, is that the inner thylakoids primarily make ATP (adenosine triphosphate), the energy currency of the cell, by cyclic electron transport around photosystem I, and the peripheral ones do linear electron flow resulting in ATP as well as reduced nicotinamide adenine dinucleotide phosphate, the carrier of reducing equivalents used for carbon dioxide fixation.Hyperspectral fluorescence imaging is computationally intensive when investigating cells with multiple fluorescing pigments, but the results thus far illustrate the great power of the technique in recognizing the detailed localization of fluorescing compounds inside small cells, Vermaas said. This work opens up new vistas for localization of several different proteins, pigments and other fluorescing compounds inside a cell, and this type of imaging is likely to become an integral tool for “systems biology,” which seeks to holistically analyze the interplay between proteins, metabolites and the energy/redox state of the cell in order to understand how cells function.
The bottom line here is that even if cell ‘compartments,’ like thylakoid membranes, cannot be distinguished in an electron microscope, there is a functional heterogeneity, because of different protein complexes in different parts of the thylakoids. This heterogeneity had long been suspected, but never been proven experimentally.
These results show that hyperspectral fluorescence imaging can provide new information regarding pigment organization and localization even in small cells, and provides a new approach in in vivo localization of complex mixtures of fluorescing compounds at high resolution. - Professor Vermaas
The work was funded by the U.S. Department of Energy.
Image: Hyperspectral imaging scans through wild-type Synechocystis cells. Credit: Vermaas, et. al., PNAS.
References:
Vermaas, W.F.J., et. al. "In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells", PNAS, Published online on March 3, 2008, DOI: 10.1073/pnas.0708090105
 --------------
--------------
 Technip has been awarded by KNM Process Systems Sdn Bhd a contract to provide assistance in the detailed engineering of the fatty acids methyl ester transesterification unit for a biodiesel production plant to be located at the port of Kuantan, Malaysia. This project will be executed by Mission Biofuel Sdn Bhd (investor), KNM (contractor) and Axens (licensor). The unit will produce 250,000 tons of biodiesel per year from palm oil. It is scheduled to go into production in the third quarter of 2008.
Technip has been awarded by KNM Process Systems Sdn Bhd a contract to provide assistance in the detailed engineering of the fatty acids methyl ester transesterification unit for a biodiesel production plant to be located at the port of Kuantan, Malaysia. This project will be executed by Mission Biofuel Sdn Bhd (investor), KNM (contractor) and Axens (licensor). The unit will produce 250,000 tons of biodiesel per year from palm oil. It is scheduled to go into production in the third quarter of 2008.









0 Comments:
Post a Comment
Links to this post:
Create a Link
<< Home